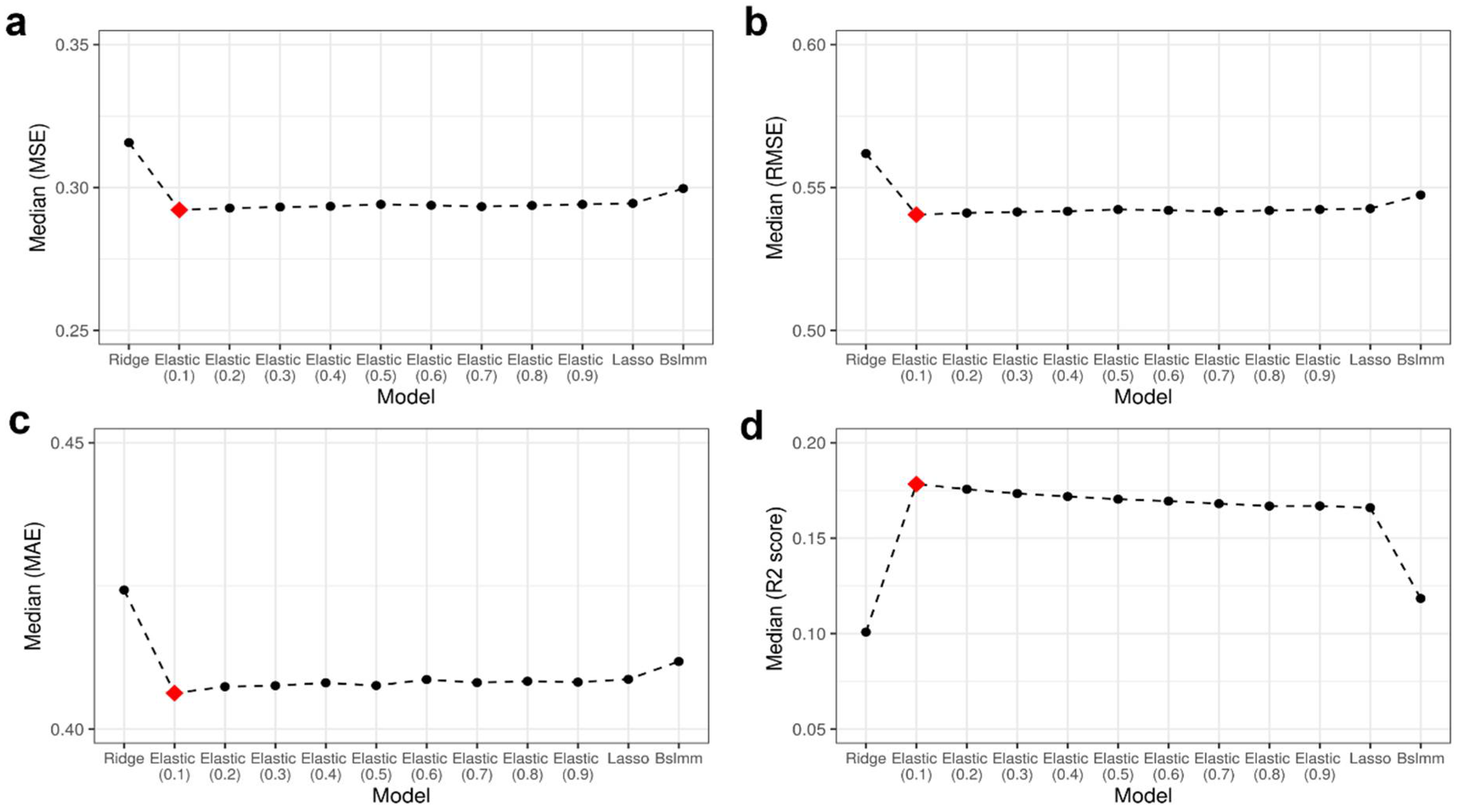
Fluctuating DNA Methylation: Unraveling the Evolutionary Tapestry of Cancer
Cancer is not a static disease; it’s a dynamic process characterized by continuous evolution. Understanding this evolution is crucial for developing effective therapies and improving patient outcomes. Recent research highlights the pivotal role of fluctuating DNA methylation in driving this evolutionary journey. This article delves into the complex relationship between DNA methylation changes and cancer progression, exploring how these epigenetic modifications contribute to the heterogeneity and adaptability of cancer cells.
What is DNA Methylation?
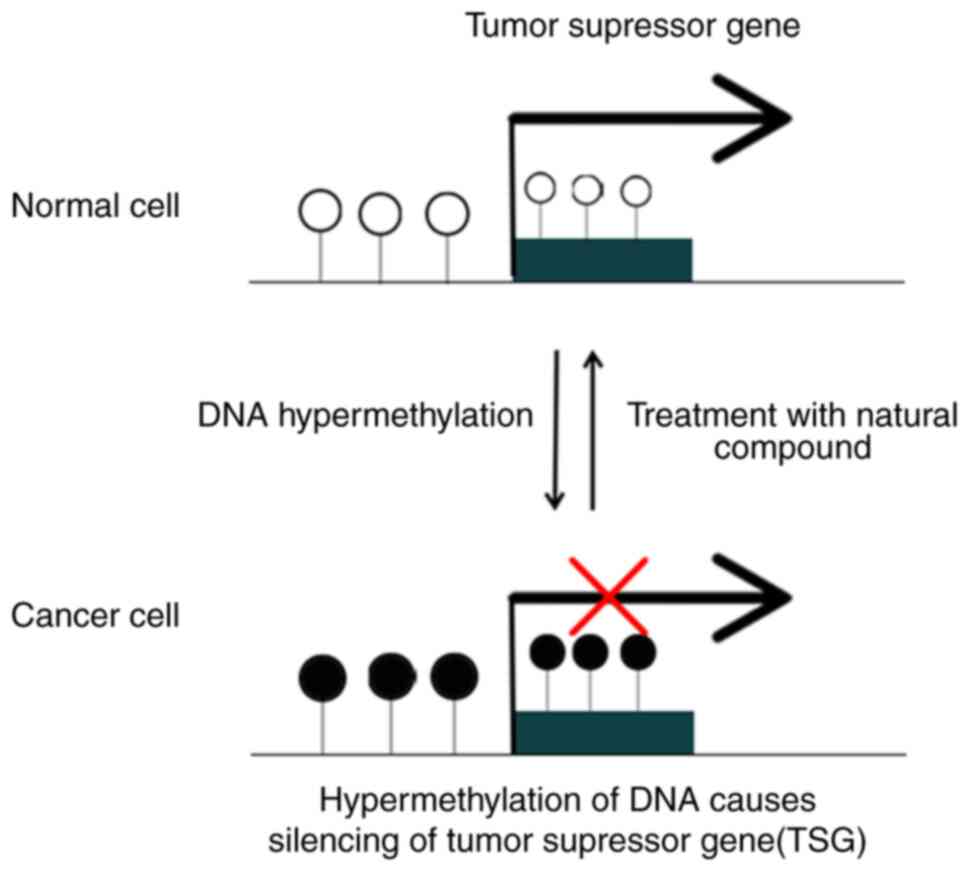
DNA methylation is an epigenetic modification, meaning it alters gene expression without changing the underlying DNA sequence. It involves the addition of a methyl group (CH3) to a cytosine base, typically within CpG islands (regions rich in cytosine-guanine dinucleotides). This modification can either silence or activate gene expression, depending on the location and context. In healthy cells, DNA methylation patterns are tightly regulated, ensuring proper gene control. However, in cancer, this regulation is often disrupted, leading to widespread methylation changes.
The Dynamic Landscape of Methylation in Cancer
Unlike previously believed, DNA methylation in cancer is not a static event. Instead, it exhibits significant fluctuation throughout the disease’s progression. This dynamic nature reflects the adaptability of cancer cells to their constantly changing environment.
- Early Stages: Initial methylation changes may involve the silencing of tumor suppressor genes, promoting uncontrolled cell growth.
- Progression: As the tumor grows, further methylation alterations occur, affecting genes involved in cell cycle regulation, apoptosis (programmed cell death), DNA repair, and immune evasion.
- Metastasis: Metastatic spread is often accompanied by dramatic shifts in DNA methylation patterns, enabling cancer cells to adapt to new microenvironments and colonize distant sites. This contributes to the heterogeneity observed in metastatic lesions.
- Treatment Response: Changes in methylation can also influence the response to cancer therapies. Some therapies target epigenetic modifications, aiming to restore normal gene expression patterns. However, the fluctuating nature of methylation presents challenges for targeted therapies.
How Fluctuating Methylation Drives Cancer Evolution
The dynamic nature of DNA methylation contributes to cancer evolution in several ways:
- Increased Genetic Instability: Aberrant methylation can disrupt DNA repair mechanisms, leading to increased mutations and genomic instability, further fueling cancer progression.
- Selection Pressure: The fluctuating methylation landscape creates a diverse population of cancer cells, some of which may possess advantageous epigenetic modifications that allow them to survive and proliferate under selective pressures, such as chemotherapy or radiotherapy.
- Epigenetic Heterogeneity: This diversity in methylation patterns contributes to the heterogeneity observed within a single tumor, making it more challenging to treat effectively. This intratumoral heterogeneity is a major factor contributing to treatment resistance and relapse.
- Immune Evasion: Alterations in methylation can affect the expression of immune-related genes, enabling cancer cells to evade detection and destruction by the immune system.
Clinical Implications and Future Directions
Understanding the dynamic nature of DNA methylation in cancer holds significant clinical implications. It highlights the need for:
- More comprehensive diagnostic approaches: Beyond traditional genomic analysis, incorporating epigenetic profiling (including DNA methylation analysis) could provide a more complete picture of tumor biology and guide treatment decisions.
- Development of novel therapies: Targeting specific methylation changes could offer new therapeutic strategies, particularly in combination with other therapies.
- Personalized medicine: The heterogeneity of methylation patterns underscores the need for personalized approaches to cancer treatment, tailored to the unique epigenetic profile of each patient’s tumor.
Conclusion
The dynamic interplay of DNA methylation alterations plays a pivotal role in the evolutionary journey of cancer. The fluctuating nature of these epigenetic modifications contributes to the heterogeneity, adaptability, and treatment resistance of cancer cells. Further research into these dynamic processes is essential for developing more effective diagnostic and therapeutic strategies to combat this complex disease.
FAQs
Q1: Can changes in DNA methylation be reversed?
A1: Yes, in some cases. Certain drugs, known as epigenetic modifiers, can target and reverse aberrant methylation patterns. However, the extent to which methylation changes can be reversed and the long-term effectiveness of these approaches are still areas of active research.
Q2: Is DNA methylation inherited?
A2: While the underlying DNA sequence is inherited, DNA methylation patterns are largely reset during gametogenesis (the formation of sperm and egg cells). However, some methylation patterns can be inherited across generations, although this is less common than previously thought. In cancer, the aberrant methylation patterns are not typically inherited but are acquired during the lifetime of the individual.
Q3: How is DNA methylation measured?
A3: Several techniques are used to measure DNA methylation, including bisulfite sequencing, methylation-specific PCR (MSP), and microarrays. These methods allow researchers to assess the methylation status of specific genes or genomic regions.
Q4: Can DNA methylation be used for early cancer detection?
A4: Research is ongoing to explore the use of DNA methylation as a biomarker for early cancer detection. Changes in methylation patterns in bodily fluids (like blood) may offer a non-invasive way to detect cancer at an early stage.
Q5: Are there any ethical considerations related to research on DNA methylation and cancer?
A5: Ethical considerations include ensuring informed consent from participants in research studies, protecting the privacy of genetic information, and addressing potential disparities in access to epigenetic-based diagnostics and therapies.