Conquer Your Chemistry Class: Mastering Bond Type Practice Worksheets
Chemistry, with its intricate dance of atoms and molecules, can seem daunting. Understanding how these tiny building blocks interact – specifically, the types of bonds that hold them together – is crucial for success. This article provides a comprehensive guide to “Bond Type Practice Worksheet Answers,” helping you navigate this essential topic and ace your exams. We’ll delve into the key bond types, explore effective study strategies, and equip you with the knowledge needed to confidently tackle any worksheet.
Understanding the Fundamentals: Types of Chemical Bonds
Before diving into worksheets, a solid grasp of the different bond types is paramount. Chemical bonds are the forces of attraction that hold atoms together, forming molecules and compounds. Here’s a breakdown of the primary bond types:
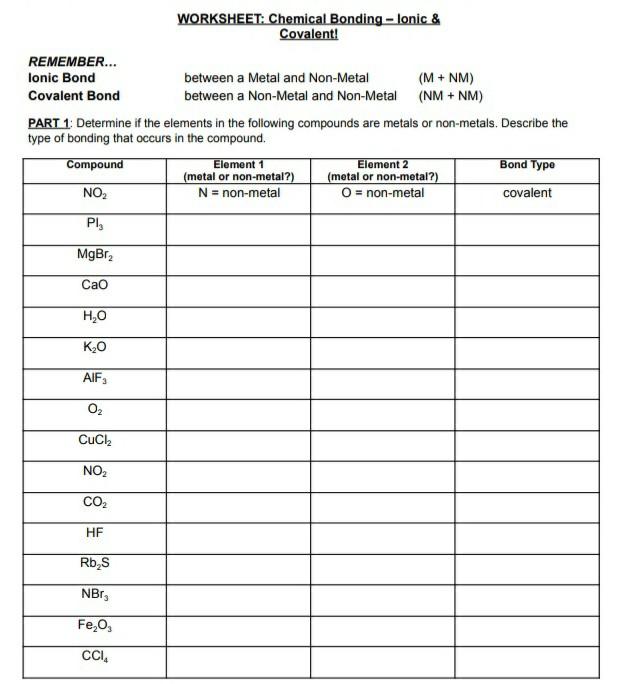
- Ionic Bonds: These bonds form through the transfer of electrons between atoms, typically a metal and a nonmetal. The resulting oppositely charged ions are held together by electrostatic attraction.
- Key Characteristics: High melting and boiling points, conducts electricity when dissolved in water (electrolytes).
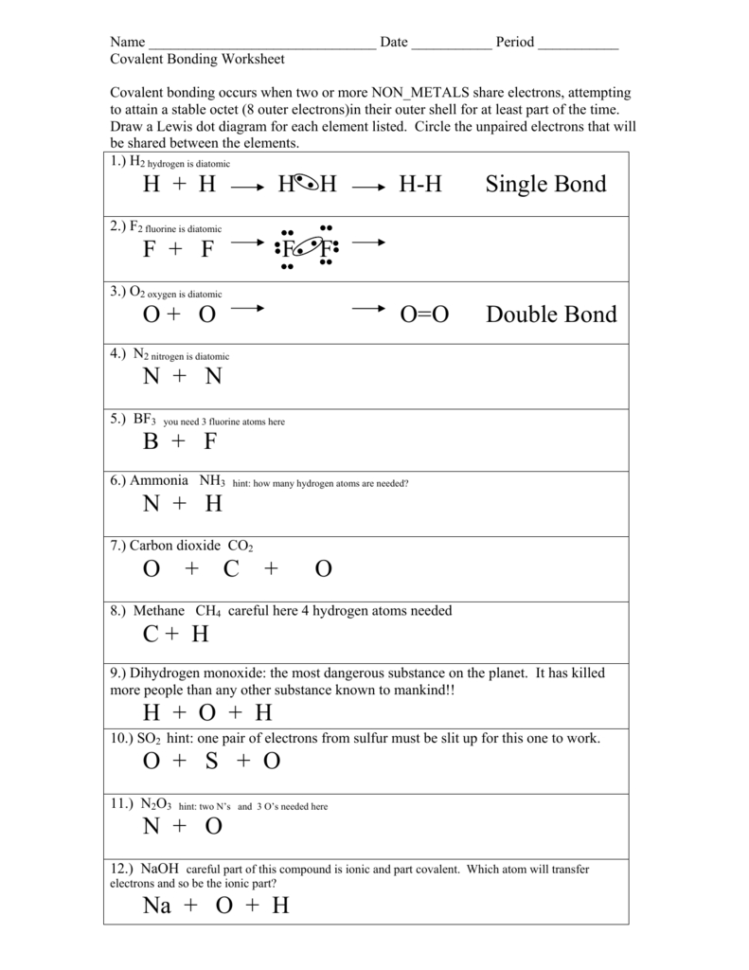
- Covalent Bonds: These bonds form through the sharing of electrons between atoms, usually nonmetals. The sharing can be equal (nonpolar) or unequal (polar).
- Key Characteristics: Lower melting and boiling points compared to ionic compounds, can exist as solids, liquids, or gases, and may or may not conduct electricity.
- Metallic Bonds: These bonds occur in metals, where valence electrons are delocalized and form a “sea” of electrons surrounding positively charged metal ions.
- Key Characteristics: Excellent conductors of electricity and heat, malleable and ductile (can be hammered into sheets and drawn into wires).
Decoding the Worksheet: Key Skills for Success
Successfully completing bond type practice worksheets requires more than just memorization. You’ll need to develop specific skills to analyze molecules and determine the bond types present. Here’s what you need to focus on:
- Electronegativity: Understanding electronegativity, a measure of an atom’s ability to attract electrons in a chemical bond, is crucial. The difference in electronegativity between two bonded atoms helps determine the bond’s polarity.
- Practice Tip: Memorize the general trends of electronegativity across the periodic table (increases from left to right and from bottom to top).
- Lewis Structures: Drawing Lewis structures (dot diagrams) helps visualize the valence electrons and the bonding patterns within a molecule. This allows you to identify which atoms are involved in bonding and how many electrons are shared or transferred.
- Practice Tip: Practice drawing Lewis structures for various molecules, including those with single, double, and triple bonds.
- Identifying Metals and Nonmetals: Recognizing the elements’ placement on the periodic table is key. Metals tend to form ionic bonds with nonmetals. Nonmetals typically form covalent bonds with each other.
- Practice Tip: Familiarize yourself with the periodic table and the general location of metals, nonmetals, and metalloids.
- Polarity vs. Nonpolarity: Understanding the difference between polar and nonpolar covalent bonds is essential. This depends on the electronegativity difference between the bonded atoms and the overall molecular geometry.
- Practice Tip: Consider the shape of the molecule. Symmetrical molecules with polar bonds can be nonpolar overall due to the cancellation of dipole moments.
Strategies for Effective Practice: Worksheet Solutions and Beyond
Simply looking at the “Bond Type Practice Worksheet Answers” isn’t enough. Effective practice involves active learning and applying the concepts. Here’s a strategic approach:
- Work Through Problems: Don’t just peek at the answers! Attempt each problem independently first. This helps solidify your understanding and pinpoint areas where you struggle.
- Analyze Your Mistakes: When you check your answers, meticulously analyze any errors. Identify the specific concept you misunderstood or the step where you went wrong.
- Seek Clarification: If you’re struggling with a particular concept, don’t hesitate to ask your teacher, classmates, or utilize online resources like Khan Academy or Chemistry LibreTexts.
- Practice Regularly: Consistent practice is key to mastering bond types. Dedicate time each day or week to work through practice problems.
- Use the Answers as a Learning Tool: Once you’ve attempted the problem, use the answer key not just to see if you got the right answer, but how the answer was derived. Understand the reasoning behind each step.
The Benefits of Mastering Bond Types
A strong understanding of bond types is foundational for advanced chemistry concepts. By mastering this topic, you’ll:
- Build a Strong Foundation: Prepare yourself for more complex topics such as chemical reactions, molecular shapes, and intermolecular forces.
- Improve Problem-Solving Skills: Develop critical thinking and analytical abilities.
- Boost Your Confidence: Gain confidence in your ability to tackle challenging chemistry problems.
- Enhance Exam Performance: Improve your grades on exams and quizzes.
Conclusion: Your Path to Bond Type Mastery
Mastering bond types is achievable with dedicated effort and a strategic approach. By understanding the fundamentals, practicing effectively, and utilizing available resources, you can conquer your chemistry worksheets and excel in your studies. Remember to focus on the underlying concepts, analyze your mistakes, and seek help when needed. With consistent practice, you’ll be well on your way to understanding the fundamental forces that hold matter together and succeeding in your chemistry journey.
Frequently Asked Questions (FAQs)
1. What is the easiest way to determine if a bond is ionic or covalent?
The easiest way is to consider the elements involved. Bonds between a metal and a nonmetal are typically ionic, while bonds between two nonmetals are typically covalent. However, the electronegativity difference between the atoms is the most accurate indicator.
2. How do I account for the shape of a molecule when determining its polarity?
Molecular shape is crucial. Even if a molecule contains polar bonds, it can be nonpolar if the bonds are arranged symmetrically, canceling out their individual polarities. For example, carbon dioxide (CO2) has polar C=O bonds, but it is a linear molecule, making it nonpolar overall.
3. Where can I find more bond type practice worksheets?
There are numerous resources available online. Search for “bond type practice worksheet” or “ionic and covalent bond practice worksheet” on Google. Your textbook and the resources provided by your teacher are also excellent sources.