UK Launches Clinical Trial to Transform Alzheimer’s Diagnosis
The fight against Alzheimer’s disease, a devastating neurological condition affecting millions, has received a significant boost with the launch of a groundbreaking clinical trial in the UK. This innovative research aims to revolutionize early diagnosis, potentially offering life-changing benefits for patients and their families. The trial focuses on a new diagnostic approach that promises faster, more accurate, and less invasive methods of detecting the disease, paving the way for earlier interventions and improved management.
A Paradigm Shift in Alzheimer’s Diagnosis
Current methods for diagnosing Alzheimer’s disease are often lengthy, expensive, and rely heavily on subjective assessments. This can lead to delays in diagnosis, hindering the timely implementation of effective treatments and support. The UK’s new clinical trial seeks to address these limitations by exploring:
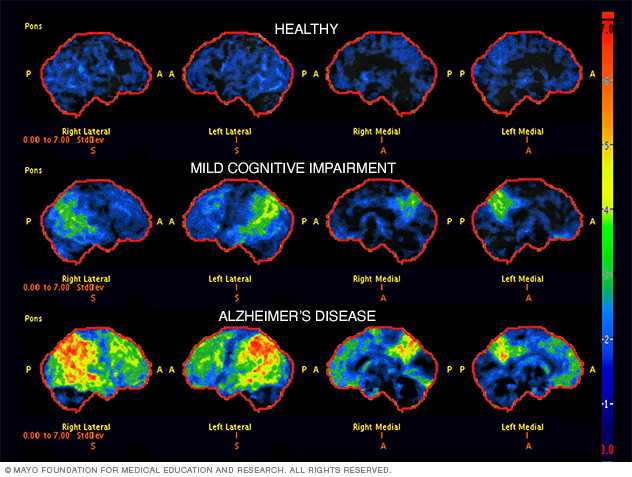
- Advanced neuroimaging techniques: The trial will utilize cutting-edge brain scans to detect subtle changes indicative of Alzheimer’s pathology far earlier than currently possible.
- Novel biomarkers: Researchers will investigate the use of novel biomarkers – specific molecules or substances found in blood or cerebrospinal fluid – that can serve as reliable indicators of the disease’s presence.
- Artificial intelligence (AI): AI algorithms will be employed to analyze the collected data, enhancing the accuracy and speed of diagnosis. This allows for the identification of patterns that might be missed by human observation alone.
The Potential Impact of Early Diagnosis
Early diagnosis of Alzheimer’s is crucial for several reasons:
- Early intervention: Starting treatment earlier can help slow disease progression and potentially improve the quality of life for patients.
- Improved care planning: An early diagnosis allows individuals and their families to plan for the future, making necessary arrangements for care and support.
- Reduced healthcare costs: Early intervention can help mitigate the long-term costs associated with managing advanced Alzheimer’s disease.
- Clinical trial participation: Early diagnosis increases the chances of participation in clinical trials testing new treatments and therapies.
The Trial’s Methodology and Participants
The clinical trial will involve a significant number of participants across various UK locations. The exact number and selection criteria will be detailed in official publications released by the research team. The trial will follow rigorous scientific protocols, ensuring the reliability and validity of the findings. Data collected during the trial will be meticulously analyzed to assess the effectiveness and accuracy of the new diagnostic approach.
The Future of Alzheimer’s Diagnosis
The UK’s ambitious clinical trial represents a significant step forward in the battle against Alzheimer’s disease. If successful, this research could lead to a paradigm shift in how the disease is diagnosed, ultimately transforming the lives of countless individuals and families affected by this devastating condition. The potential for earlier and more accurate diagnosis holds immense promise for improving patient outcomes and fostering advancements in treatment and care.
Frequently Asked Questions (FAQs)
Q: How long will the clinical trial last? A: The duration of the trial will be determined by the research team and will depend on several factors, including participant recruitment and data analysis. Specific timelines will be published as the trial progresses.
Q: Who is eligible to participate in the trial? A: Eligibility criteria will be clearly defined by the research team and will likely involve specific age ranges, cognitive assessments, and other relevant factors. Information on eligibility will be available through official channels related to the trial.
Q: What are the risks involved in participating in the trial? A: While the trial aims to minimize risks, participation in any clinical trial carries inherent risks. Potential participants will receive detailed information about the risks and benefits before consenting to participate.
Q: Where can I find more information about the trial? A: Further information about the trial, including updates on progress and results, will be published on the relevant research institution’s website and through other official channels.
Q: Will the results of the trial be made publicly available? A: The results of the trial are expected to be published in peer-reviewed scientific journals and made available to the public, contributing to the broader understanding and management of Alzheimer’s disease.